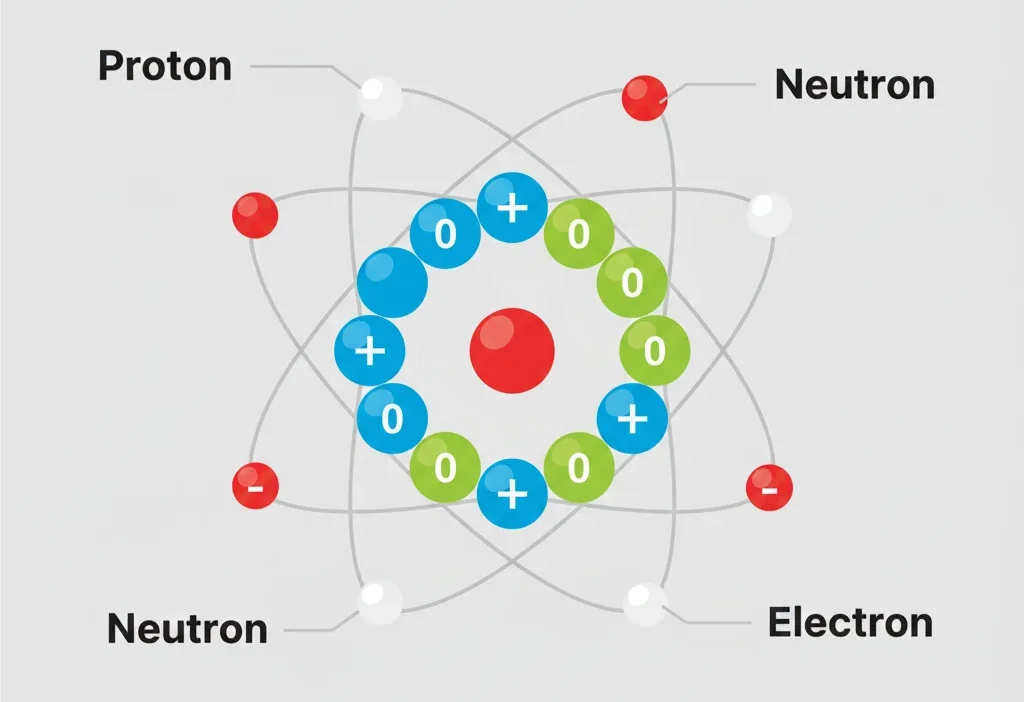
👉 Neutrons are neither positive nor negative. They have no electric charge.
That’s it. Protons are positive, electrons are negative, and neutrons are neutral — like the referee in a game who takes no side.
Now let’s break this down slowly, clearly, and in a way even a 4th-grade student can easily understand.
Many students and beginners ask the same question again and again:
“Are neutrons positive or negative?”
This confusion is very common — and very normal. Why? Because neutrons are always taught together with protons and electrons. When we hear:
- Protons are positive
- Electrons are negative
Our brain automatically asks:
👉 So… what about neutrons?
In this easy guide, you will learn:
- What neutrons really are
- Whether neutrons are positive, negative, or neutral
- The key difference between neutrons, protons, and electrons
- Simple examples from daily life
- Common mistakes and how to avoid them
Don’t worry. No hard science words. No confusion.
By the end, you will fully understand neutrons — clearly and confidently.
What Is a Neutron? (Simple Meaning)
Meaning of Neutron (In Easy Words)
A neutron is a tiny particle found inside the center of an atom.
An atom has three main particles:
- Protons
- Electrons
- Neutrons
Neutrons live in the nucleus (center) of the atom, right next to protons.
What Charge Does a Neutron Have?
👉 Neutrons have NO electric charge.
They are:
- ❌ Not positive
- ❌ Not negative
- ✅ Neutral
That is why they are called neutrons (from the word neutral).
Easy Examples to Remember
- Think of protons as “+” signs.
- Think of electrons as “–” signs.
- Think of neutrons as “0” — nothing, neutral.
Mini Story (For Easy Memory)
Imagine a family:
- Father = positive (+) → Proton
- Mother = negative (–) → Electron
- Child = neutral (0) → Neutron
The child doesn’t take sides.
That’s exactly what a neutron does.
What Are Protons and Electrons? (Quick Reminder)
To clearly understand are neutrons positive or negative, we must compare them with the other two particles.
Proton
- Charge: Positive (+)
- Location: Inside the nucleus
- Role: Gives the atom its identity (element)
Electron
- Charge: Negative (–)
- Location: Moves around the nucleus
- Role: Helps in electricity and bonding
Neutron
- Charge: Neutral (0)
- Location: Inside the nucleus
- Role: Adds weight and stability
The Key Difference Between Neutrons, Protons, and Electrons
Comparison Table (Simple & Visual)
| Particle | Charge | Location in Atom | Easy Example |
|---|---|---|---|
| Proton | Positive (+) | Inside nucleus | Plus sign ➕ |
| Electron | Negative (–) | Outside nucleus | Minus sign ➖ |
| Neutron | Neutral (0) | Inside nucleus | Zero ⚪ |
⭐ Quick Tip to Remember
PEN Rule
- Proton = Positive
- Electron = Negative
- Neutron = Neutral
Common Mistakes About Neutrons (And How to Fix Them)
❌ Mistake 1: “Neutrons are negative”
✅ Correct: Neutrons are neutral.
Why this happens:
Students mix neutrons with electrons.
❌ Mistake 2: “Neutrons have a small positive charge”
✅ Correct: Neutrons have zero charge.
Why this happens:
People think all nucleus particles must be positive.
❌ Mistake 3: “Neutrons don’t matter”
✅ Correct: Neutrons are very important.
They:
- Add mass to atoms
- Help keep the nucleus stable
When Do We Talk About Neutrons? (Real-Life Use)
We talk about neutrons when learning:
- Atoms and elements
- Basic physics or chemistry
- Nuclear energy
- Science exams
Easy Sentences Using “Neutron”
- A neutron has no electric charge.
- Neutrons are found inside the nucleus.
- Hydrogen has no neutron.
- Heavy atoms have more neutrons.
- Neutrons help hold the nucleus together.
Are Neutrons Positive or Negative? (Clear Final Answer)
Let’s say it again — clearly and confidently:
👉 Neutrons are NOT positive.
👉 Neutrons are NOT negative.
👉 Neutrons are NEUTRAL.
If a question asks:
Are neutrons positive or negative?
The correct answer is:
Neither. Neutrons have no charge.
Memory Hack (So You Never Forget)
🧠 Traffic Light Trick
- Red = Positive → Proton
- Green = Negative → Electron
- Yellow = Neutral → Neutron (stay in the middle)
Quick Recap: Neutrons vs Protons vs Electrons
- Neutrons have no charge
- Protons are positive
- Electrons are negative
- Neutrons sit quietly in the nucleus
- Neutrons add weight and stability
Advanced Tips (Optional but Helpful)
History of the Neutron
- Neutron was discovered in 1932 by James Chadwick
- It helped scientists understand atomic mass
In Exams & Formal Writing
Always write:
“Neutrons are electrically neutral particles found in the nucleus.”
In Online or Text Writing
Wrong info spreads fast.
Always remember: neutral = zero charge.
Mini Quiz: Test Yourself ✅
Fill in the blanks:
- A neutron has ___ charge.
- Protons are ___ in charge.
- Electrons are ___ in charge.
- Neutrons are found inside the ___.
- The PEN rule helps remember ___, ___, and ___.
Answers:
- No / zero
- Positive
- Negative
- Nucleus
- Proton, Electron, Neutron
Conclusion
Now you clearly know the answer to the common question:
Are neutrons positive or negative?
Neutrons are neutral. They carry no electric charge.
They quietly sit in the nucleus, helping atoms stay strong and stable.
Understanding this small idea makes science much easier.
Keep learning. Keep asking questions.
Every day, your knowledge grows stronger — just like atoms with neutrons inside them.
FAQs
1. Are neutrons positive or negative?
Neutrons are neither positive nor negative. They are neutral.
2. Why are neutrons neutral?
Because they do not carry any electric charge.
3. Where are neutrons found?
Inside the nucleus of an atom.
4. Do neutrons affect electricity?
No. Only electrons affect electricity.
5. Are neutrons important?
Yes. They add mass and stability to atoms.

Jenn Ashworth offers clear, engaging explanations of language and usage, helping readers grasp meanings, nuances, and differences with accuracy and ease.