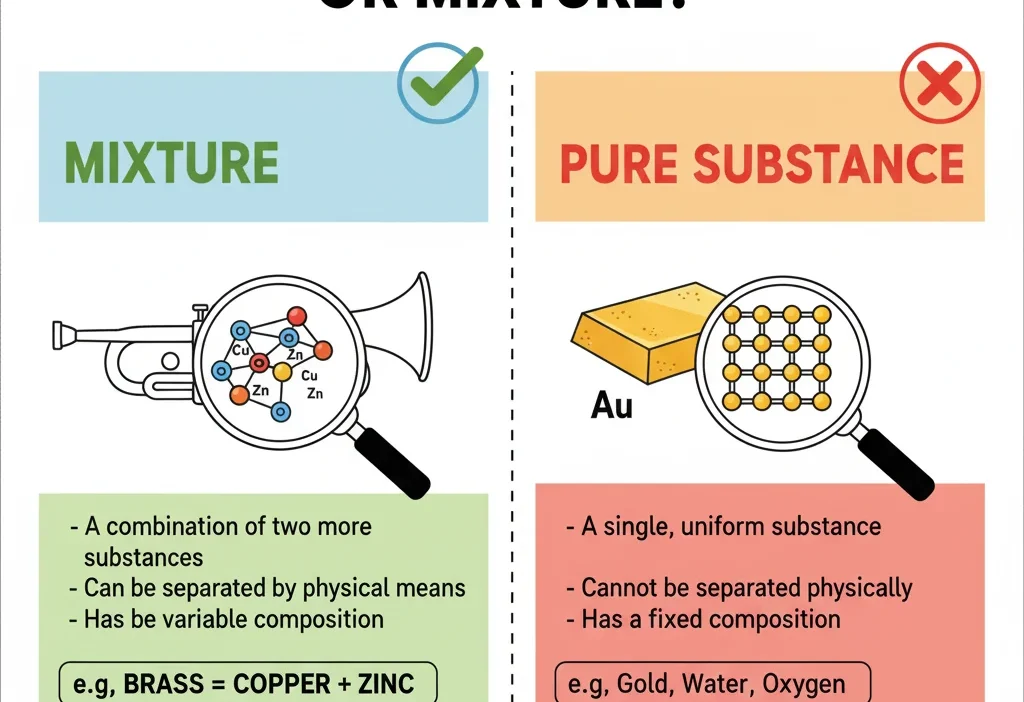
Brass is a mixture, not a pure substance. It is an alloy made primarily of copper and zinc, and sometimes other metals, which means it combines multiple elements without forming a chemical compound.
Have you ever wondered if brass is a pure substance or a mixture? 🤔 You’re not alone! Many students, hobbyists, and even beginners in science mix these terms up. Understanding the difference is crucial, especially in chemistry, metalworking, or school experiments.
In this guide, you’ll learn exactly what brass is, why it’s classified as a mixture, and how it differs from pure substances. We’ll use simple examples, real-life comparisons, and even mini-tips to make it super easy. By the end, you’ll confidently answer: “Is brass a pure substance or mixture?” — every time!
What Does Each Term Mean?
Before we dive into brass, let’s quickly understand pure substances and mixtures.
1. Pure Substance
A pure substance contains only one type of element or compound. Its composition is uniform, meaning every part of it is the same.
Part of Speech: Noun
Examples:
- Water (H₂O) — every molecule is identical.
- Gold (Au) — only gold atoms, no other metals.
- Oxygen (O₂) — a single element, pure gas.
Memory Tip: Think of pure substances like a glass of milk with nothing added — consistent and the same everywhere.
2. Mixture
A mixture combines two or more substances physically, without chemical bonding. Each part keeps its original properties.
Part of Speech: Noun
Examples:
- Salad — you can see and separate each ingredient.
- Saltwater — salt dissolves but can be filtered or evaporated.
- Brass — copper and zinc mixed together.
Memory Tip: Mixtures are like trail mix — all ingredients together but still separate.
The Key Difference Between Pure Substances and Mixtures
| Feature | Pure Substance | Mixture |
|---|---|---|
| Composition | Only one type of element/compound | Two or more substances physically combined |
| Properties | Uniform throughout | Properties of each component are retained |
| Separation | Can’t be separated physically | Can often be separated physically |
| Example | Water, gold, oxygen | Brass, salad, saltwater |
Quick Tip: If you can physically separate the components, it’s a mixture.
Common Mistakes and How to Avoid Them
Many beginners assume brass is pure because it looks uniform and shiny. Here’s how to avoid the confusion:
Mistake 1: “Brass is a pure substance because it is metallic.”
Correction: Brass is a mixture of copper and zinc. Its metallic shine doesn’t make it pure.
Mistake 2: “All metals are pure substances.”
Correction: Many metals we use daily, like brass or bronze, are alloys (mixtures). Only elements like gold or silver can be pure.
Mistake 3: “Mixtures always look mixed.”
Correction: Some mixtures like brass look uniform (homogeneous), but they are still mixtures.
When to Use “Pure Substance”
Use this term when referring to materials made of a single type of element or compound.
Examples in Real Life:
- “Gold jewelry is a pure substance if it is 24K.”
- “Oxygen in a laboratory cylinder is a pure substance.”
- “Distilled water is a pure substance.”
- “Sulfur powder from a chemical supplier is a pure substance.”
Memory Hack: If the material cannot be separated physically, call it a pure substance.
When to Use “Mixture”
Use “mixture” when a material contains more than one substance physically combined.
Examples in Real Life:
- “Brass is a mixture of copper and zinc.”
- “Saltwater is a mixture, not pure water.”
- “Trail mix is a tasty mixture of nuts and raisins.”
- “Concrete is a mixture of sand, gravel, and cement.”
Quick Recap: Pure Substance vs Mixture ✅
- Pure Substance: Only one element or compound, uniform, can’t separate physically.
- Mixture: Two or more substances, combined physically, can often separate.
- Brass: A mixture (alloy of copper and zinc).
Easy Rule to Remember: “If it can be separated physically → mixture; if not → pure substance.”
Advanced Tips (Optional)
- Origin of the terms: “Substance” comes from Latin substantia, meaning “essence.” “Mixture” comes from mixtura, meaning “mixing.”
- Formal Writing: In essays or science exams, always specify that alloys like brass are homogeneous mixtures, even though they look uniform.
- Online Misuse: Sometimes people casually call brass a “metal” without clarifying mixture vs pure substance — this can mislead students.
Soil Grass Biotic or Abiotic – What Scientists Don’t Tell You
Mini Quiz: Test Yourself! 📝
Fill in the blanks:
- Brass is a ________ because it contains copper and zinc.
- Gold is a ________ substance.
- Saltwater is a ________ of salt and water.
- Pure substances ________ be separated physically.
- Mixtures ________ be separated physically.
Answers: 1. Mixture 2. Pure 3. Mixture 4. Cannot 5. Can
Conclusion
Now you know the clear difference between pure substances and mixtures. Brass is a mixture, even though it looks like a single metal. Remember, the key is whether the components can be physically separated. Practice spotting mixtures and pure substances in your daily life — metals, drinks, and even snacks!
Learning this small but important science rule will make chemistry, schoolwork, and even casual science conversations much easier. Keep exploring and improving your understanding every day!
FAQs
1. Is brass a pure metal?
No, brass is an alloy, which means it’s a mixture of copper and zinc.
2. Can brass be separated into copper and zinc?
Yes, but only through special chemical or physical processes, not by simple separation.
3. Why does brass look like a pure substance?
Because it is homogeneous — all parts look the same even though it’s a mixture.
4. Are all alloys mixtures?
Yes, alloys like bronze, steel, and brass are mixtures of two or more metals.
5. What is the easiest way to remember if a material is a mixture or pure?
Ask: Can you separate it physically? If yes → mixture. If no → pure substance.

Francis Sufford crafts thoughtful, insightful explanations on language, meaning, and usage, blending clarity with storytelling to guide readers effectively.