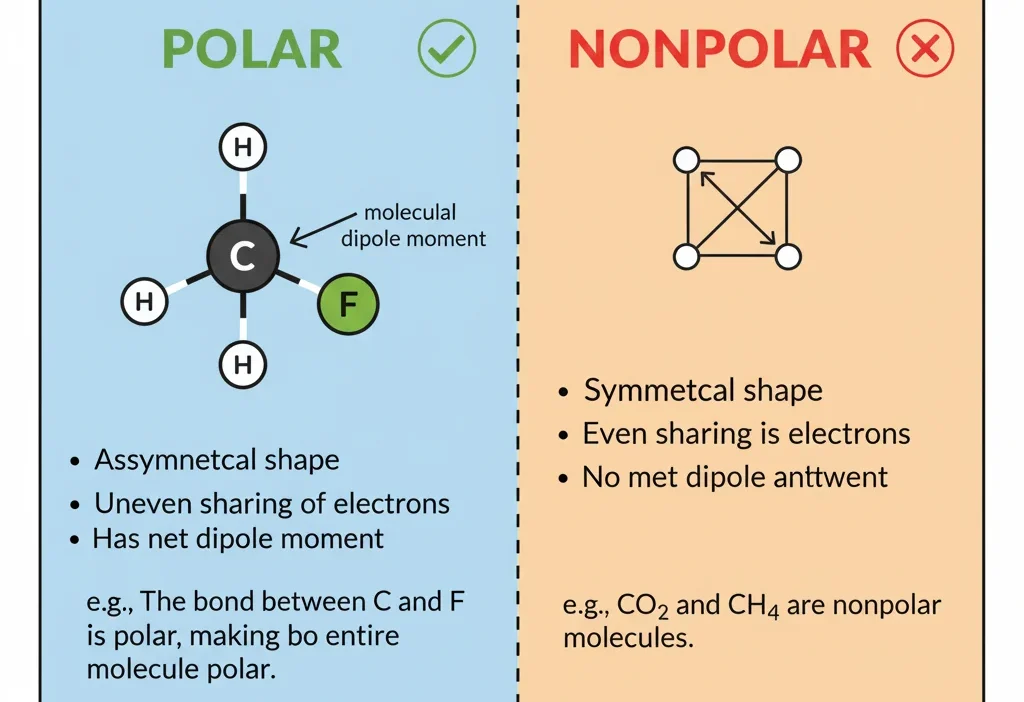
CH3F is polar due to its asymmetrical shape and the electronegativity difference between carbon, hydrogen, and fluorine atoms. The molecule has a net dipole moment, making it polar.
Ever wondered whether CH3F is polar or nonpolar? Many students and chemistry enthusiasts get confused when looking at small molecules like CH3F (fluoromethane). Understanding polarity is crucial because it affects solubility, boiling points, and chemical reactions.
In this guide, you’ll learn:
- What polarity really means.
- Why CH3F is polar, not nonpolar.
- How to predict polarity in similar molecules.
By the end of this article, even beginners will confidently understand CH3F’s polarity and use this knowledge in class, exams, or everyday science discussions.
What Does Polar and Nonpolar Mean? ⚖️
Before we focus on CH3F, let’s understand the basics:
1. Polar Molecules
- Meaning: Molecules with an uneven distribution of electrons, creating positive and negative ends (dipoles).
- Part of Speech: Adjective
- Examples:
- Water (H2O) has a bent shape, making it polar.
- Ammonia (NH3) has a net dipole due to nitrogen’s electronegativity.
- Hydrogen chloride (HCl) has one end positive, one end negative.
2. Nonpolar Molecules
- Meaning: Molecules where electrons are evenly shared, no net dipole moment.
- Part of Speech: Adjective
- Examples:
- Methane (CH4) has symmetrical bonds, making it nonpolar.
- Carbon dioxide (CO2) has linear geometry and cancels dipoles.
- Oxygen (O2) is a simple diatomic nonpolar molecule.
Tip: Think of polarity like a tug-of-war: if one side “pulls” more electrons, the molecule is polar.
The Key Difference Between Polar and Nonpolar Molecules
| Feature | Polar Molecule | Nonpolar Molecule |
|---|---|---|
| Electron Distribution | Uneven | Even |
| Dipole Moment | Yes | No |
| Example | H2O, NH3, CH3F | CH4, CO2, O2 |
| Solubility | Dissolves in water | Dissolves in oils |
| Quick Tip | Look for electronegativity differences and asymmetry | Symmetry often = nonpolar |
Quick Tip: If a molecule has polar bonds but is symmetrical, it may still be nonpolar.
Soil Grass Biotic or Abiotic – What Scientists Don’t Tell You
Why CH3F is Polar ✅
CH3F (fluoromethane) has one fluorine atom attached to a carbon with three hydrogens.
Reasons it’s polar:
- Electronegativity Difference: Fluorine is highly electronegative, while carbon and hydrogen are less so. This creates a strong dipole.
- Molecular Shape: CH3F has a tetrahedral geometry. The fluorine’s pull does not cancel out due to asymmetry.
- Net Dipole Moment: The molecule has a positive end near hydrogens and a negative end near fluorine.
Example Sentences:
- CH3F dissolves in polar solvents like water because it’s polar.
- In the lab, CH3F exhibits dipole-dipole interactions due to polarity.
Common Mistakes About CH3F Polarity ❌
| Incorrect | Correct | Explanation |
|---|---|---|
| CH3F is nonpolar | CH3F is polar | Symmetry misconception: tetrahedral shape is not perfectly symmetrical in terms of polarity |
| Fluorine makes CH3F nonpolar | CH3F is polar | Electronegativity difference creates a dipole, making it polar |
Tip: Always check both electronegativity difference and molecular geometry.
When to Use the Concept of Polar vs Nonpolar
1. Predicting Solubility:
- CH3F mixes with polar solvents but not with nonpolar oils.
Example: “CH3F dissolves in water but not in hexane.”
2. Chemical Reactions:
- Polar molecules interact with other polar molecules via dipole-dipole forces.
Example: “CH3F reacts differently than CH4 in substitution reactions.”
3. Laboratory Experiments:
- Knowing polarity helps in choosing correct solvents or predicting boiling points.
Quick Recap: CH3F Polarity Explained 🌈
- CH3F is polar.
- Polar molecules have uneven electron distribution → net dipole.
- Nonpolar molecules share electrons evenly → no dipole.
- Check both bond type and geometry to determine polarity.
- Solubility, reactivity, and intermolecular forces depend on polarity.
Advanced Tips (Optional) 🧪
- History: Fluoromethane (CH3F) was first synthesized in the early 20th century as a refrigerant.
- Formal Writing: In essays, specify “CH3F is polar due to its tetrahedral geometry and electronegativity difference.”
- Online Writing: Avoid simply writing “CH3F polar?” — always clarify for better scientific accuracy.
Mini Quiz: Test Your Knowledge ✏️
Fill in the blanks:
- CH3F is ______ due to its tetrahedral shape.
- A molecule with evenly shared electrons is called ______.
- Fluorine’s high ______ pulls electrons toward itself.
- Water (H2O) is ______ because of its bent shape.
- Symmetrical molecules like CO2 are ______.
Conclusion
Now you know that CH3F is polar, why it’s polar, and how polarity affects solubility and chemical behavior. By understanding the electronegativity difference and molecular geometry, you can confidently predict the polarity of other molecules too.
Keep practicing with small molecules and soon, determining polarity will become second nature. Remember, every small step in understanding chemistry brings you closer to mastery!
FAQs
Q1: Is CH3F polar or nonpolar?
A: CH3F is polar due to its tetrahedral geometry and electronegativity difference between carbon, hydrogen, and fluorine.
Q2: Why does CH3F have a dipole moment?
A: Because the fluorine atom pulls electrons more strongly than hydrogen, creating an uneven electron distribution.
Q3: Can CH3F dissolve in water?
A: Yes, CH3F is polar, so it dissolves in polar solvents like water.
Q4: Is CH4 polar like CH3F?
A: No, CH4 is nonpolar because it is symmetrical and the bond dipoles cancel out.
Q5: How do you determine if a molecule is polar?
A: Check the electronegativity difference and molecular shape. Uneven electron distribution → polar.

Eley Williams writes clear, engaging guides on confusing words and phrases, helping readers understand meanings, differences, and correct usage with ease.