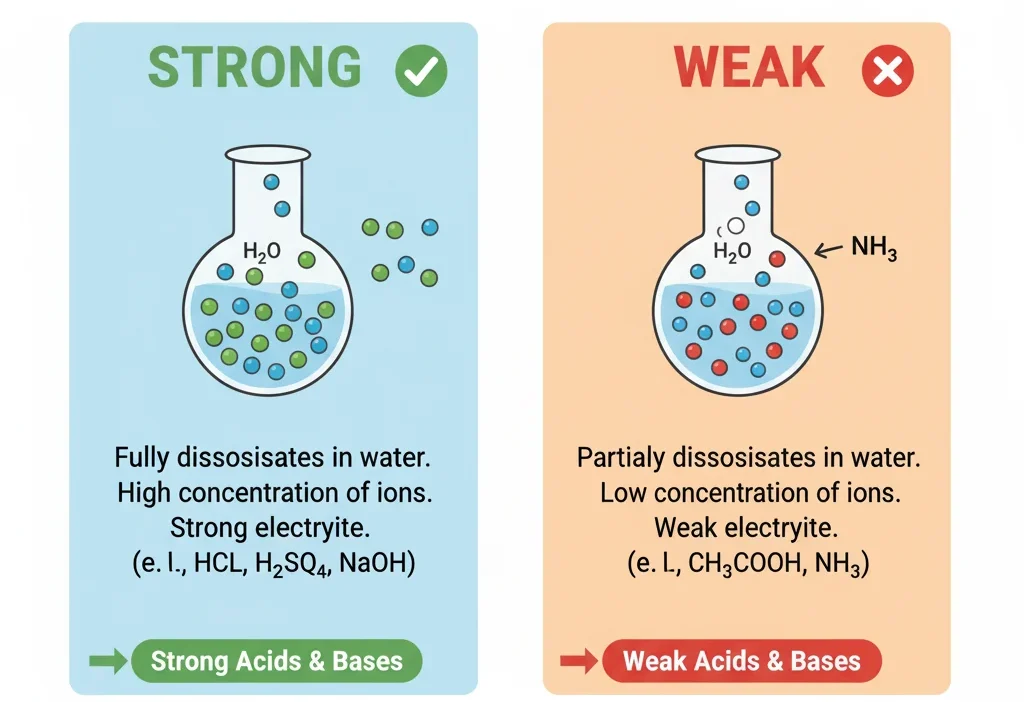
NH₃ (ammonia) is a weak base because it does not completely break apart in water. Only a small portion of NH₃ accepts hydrogen ions, so it stays mostly undissociated.
Is NH₃ strong or weak? This is one of the most confusing questions for chemistry beginners. Students often mix it up because ammonia behaves like a base, but not all bases are equally strong. Some break apart fully in water. Others only break apart a little. NH₃ falls into the second group, which makes many students unsure about its correct place.
In this simple guide, you will learn what NH₃ really is, why it’s considered weak, how it reacts in water, and how to remember the difference easily. Every explanation is written in clear, friendly English—easy enough for even a 4th-grade student to understand. Let’s break it down step by step!
What Does NH₃ Mean?
NH₃ is the chemical formula for ammonia.
It is a weak base, a colorless gas with a strong smell, and a very important compound used in fertilizers and cleaners.
What NH₃ Actually Does in Water
A base is a substance that accepts hydrogen ions (H⁺).
When NH₃ is dissolved in water, it reacts like this:
NH₃ + H₂O ⇌ NH₄⁺ + OH⁻
But here’s the key point…
Only a small amount of NH₃ turns into NH₄⁺ (ammonium) and OH⁻.
Most of it stays NH₃, unchanged.
That is why it is called a weak base.
Easy Examples Related to NH₃
- “Ammonia in cleaners has a strong smell, but it is still a weak base.”
- “NH₃ does not break apart fully in water.”
- “A weak base like NH₃ accepts only a few hydrogen ions.”
What Does “Strong” Mean in Chemistry?
Before learning why NH₃ is weak, you must understand what “strong” means in chemistry.
A strong base:
- Completely breaks apart in water
- Produces many OH⁻ (hydroxide) ions
- Has a high pH
- Examples: NaOH, KOH
A weak base:
- Only partially breaks apart
- Produces fewer OH⁻ ions
- Lower pH than strong bases
- Example: NH₃
3 Simple Examples of Strong Bases
- NaOH → Na⁺ + OH⁻
- KOH → K⁺ + OH⁻
- Ca(OH)₂ → Ca²⁺ + 2OH⁻
The Key Difference Between “Strong NH₃” and “Weak NH₃”
Even though people search for “strong NH₃,” there is no such thing.
NH₃ is always a weak base.
Below is a simple comparison:
Comparison Table: Strong Bases vs NH₃ (Weak Base)
| Feature | Strong Base (e.g., NaOH) | NH₃ (Ammonia) |
|---|---|---|
| Breaks apart in water | 100% (complete) | Very little (partial) |
| Strength | Strong | Weak |
| OH⁻ ions produced | A lot | Few |
| pH | Very high | Moderate |
| Ionization | Full | Partial |
| Example | NaOH, KOH | NH₃ |
Quick Tip to Remember
If it doesn’t break apart completely → It’s weak.
NH₃ does NOT break apart fully → NH₃ = weak base.
Common Mistakes and How to Avoid Them
❌ Mistake 1: Thinking NH₃ is strong because its smell is strong
The smell has nothing to do with chemical strength.
✔️ Correct: NH₃ is a weak base, even though the smell is strong.
❌ Mistake 2: Believing all cleaners contain strong bases
Not true.
✔️ Correct: Many cleaners contain weak bases like ammonia.
❌ Mistake 3: Confusing “strong base” with “dangerous”
Something can be weak and still dangerous.
✔️ Correct: NH₃ is weak in water but still harmful to breathe.
When to Use the Term “Strong Base”
A base is called strong when:
- It fully breaks into ions
- It produces many OH⁻ ions
- Its reaction goes to completion
Examples of Correct Use
- “NaOH is a strong base because it completely breaks into ions.”
- “KOH raises pH quickly because it is a strong base.”
- “Strong bases are fully ionized in water.”
When to Use the Term “Weak Base” (Like NH₃)
Use “weak base” when the substance only partially ionizes.
NH₃ fits this rule because:
- Only a small portion of NH₃ becomes NH₄⁺ and OH⁻
- The equilibrium does not go fully to the right
- Most NH₃ molecules stay the same
Example Sentences
- “NH₃ is a weak base used in fertilizers.”
- “Ammonia only produces a small number of OH⁻ ions.”
- “Students learn that NH₃ is the most common weak base.”
- “Weak bases do not fully ionize in water.”
- “NH₃ forms ammonium ions only in small amounts.”
Memory Hacks to Remember NH₃ Is Weak
Hack #1: Think of NH₃ as ‘Not Heavy 3’
NH₃ doesn’t “hit hard” like strong bases.
So remember: Not Heavy = Not Strong
Hack #2: “Weak Because It’s Shy”
NH₃ does not fully break apart—it’s “shy,” so it stays mostly the same.
Hack #3: Picture a Tap
Strong base = tap fully open
Weak base (NH₃) = tap barely open
Quick Recap: NH₃ Strong or Weak?
- NH₃ is a weak base
- It does not completely ionize in water
- Only a little NH₃ becomes NH₄⁺ + OH⁻
- Strong bases fully break apart
- NH₃ is weak even though it smells strong
Advanced Tips (Optional but Helpful)
1. Chemical Origin
NH₃ is made of nitrogen + hydrogen.
It forms ammonium (NH₄⁺) only when it picks up a hydrogen ion.
2. Usage in Exams
In chemistry questions, NH₃ is always written as a weak base.
3. Usage in Real Life
- Used in fertilizers
- Used in window cleaners
- Found in many household chemicals
4. Online Misuse
People often say “strong ammonia” meaning “strong smell,”
but chemically, NH₃ is still weak.
Mini Quiz: Test Your Understanding
Fill in the blanks:
- NH₃ is a ______ base.
- A strong base breaks apart ______ in water.
- NH₃ forms ______ and OH⁻ in water.
- Weak bases produce ______ ions.
- NH₃ is weak because it does not ______ completely.
- NaOH is an example of a ______ base.
- NH₃ stays mostly ______ in water.
(Answers: weak, completely, NH₄⁺, few, ionize, strong, unchanged)
Conclusion
Now you clearly understand whether NH₃ is strong or weak—and why. NH₃ is always a weak base because it only partially ionizes in water. Strong bases break apart fully, but NH₃ does not. By learning this simple difference, you can avoid common mistakes that many students make. Keep practicing, keep learning, and chemistry will get easier every day!
FAQs
1. Why is NH₃ a weak base?
Because it only partially ionizes in water and produces fewer OH⁻ ions.
2. Is NH₃ dangerous even if it’s weak?
Yes. Weak base does not mean harmless. NH₃ gas is irritating.
3. Is ammonium (NH₄⁺) strong?
No. NH₄⁺ is the conjugate acid of NH₃ and is also weak.
4. Does NH₃ raise the pH of water?
Yes, but only a little compared to strong bases.
5. Can NH₃ ever be a strong base?
No. NH₃ is always classified as a weak base in chemistry.

Polly Clark creates clear, insightful guides on language and usage, helping readers understand meanings, differences, and nuances with clarity and confidence.